CO2 Storage in Novel CO2-H2O Phases at High Pressure
An effective method for capturing and storing carbon dioxide in order to reduce atmospheric concentration is currently a research area of high interest. We investigated the potential of CO2 storage in H2O compounds by using optical Raman spectroscopy to analyze the high-pressure behavior of a CO2-H2O system in-situ. Between 26.9 and 1.0 GPa, we observed a new CO2-H2O compound whose vibrational spectra differed dramatically from pure CO2 and H2O. When pressure was decreased to 1 GPa, the CO2 vibrons and OH stretch reverted to those expected for pure CO2 and H2O respectively, indicating a CO2-H2O mixture rather than a CO2-H2O molecular compound. Based on the difference in spectra seen upon pressure cycling, as well as the difference in spectra seen at multiple locations inside the sample, we concluded that multiple forms of the CO2-H2O compounds may exist. Our results indicate that the initial structure and composition may play a role in compound formation. These novel materials have potential implications for increasing our understanding of CO2 interactions with potential host materials for improved CO2 storage applications. This research was undertaken as part of Montgomery Blair High School's Senior Research Project (SRP) Program from spring 2008 to summer 2009.This project was submitted to various high school research competitions, including Junior Science and Humanities Symposia Program, Siemens Competition, Montgomery County Science Fair, and Intel Science Talent Search.
The project was awarded:
2009 Intel Science Talent Search, Semifinalist
2009 Montgomery County Science Fair Senior Division in Chemistry, 3rd place ($50)
2009 American Society of Safety Engineers Senior Division, Winner ($500)
Collaborators
Dr. Cheng-Sheng Zha
Dr. Ho-Kwang (Dave) Mao
Carnegie Institute of Washington
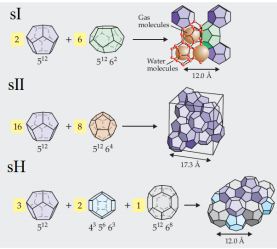
Various types of clathrate formations, depending on the constituent molecules.
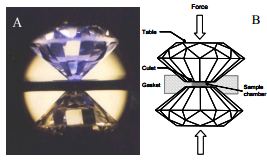
Diamond Anvil Cell used to sustain high pressures.
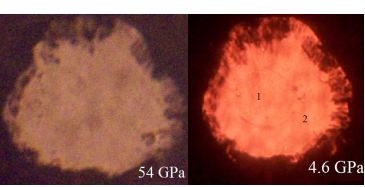
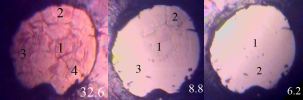
Cell sample images at various pressures.
Dr. Ho-Kwang (Dave) Mao
Carnegie Institute of Washington
Downloads
Paper PDF Presentation PDF Poster PDFPictures
Various types of clathrate formations, depending on the constituent molecules.

Diamond Anvil Cell used to sustain high pressures.

Cell sample images at various pressures.

